Chapter Assessment Reaction Rates Study Guide
- Tax Knowledge Assessment Study Guide
- Health Assessment Study Guide
- Chapter Assessment Reaction Rates Study Guided
. Define chemical reaction rate. Derive rate expressions from the balanced equation for a given chemical reaction. Calculate reaction rates from experimental data A rate is a measure of how some property varies with time. Speed is a familiar rate that expresses the distance traveled by an object in a given amount of time. Wage is a rate that represents the amount of money earned by a person working for a given amount of time.
Likewise, the rate of a chemical reaction is a measure of how much reactant is consumed, or how much product is produced, by the reaction in a given amount of time. The rate of reaction is the change in the amount of a reactant or product per unit time. Reaction rates are therefore determined by measuring the time dependence of some property that can be related to reactant or product amounts. Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in volume or pressure. For reactions involving one or more colored substances, rates may be monitored via measurements of light absorption. For reactions involving aqueous electrolytes, rates may be measured via changes in a solution’s conductivity.
For reactants and products in solution, their relative amounts (concentrations) are conveniently used for purposes of expressing reaction rates. If we measure the concentration of hydrogen peroxide, H 2O 2, in an aqueous solution, we find that it changes slowly over time as the H 2O 2 decomposes, according to the equation. This mathematical representation of the change in species concentration over time is the rate expression for the reaction.
The brackets indicate molar concentrations, and the symbol delta (Δ) indicates “change in.” Thus, represents the molar concentration of hydrogen peroxide at some time t 1; likewise, represents the molar concentration of hydrogen peroxide at a later time t 2; and ΔH 2O 2 represents the change in molar concentration of hydrogen peroxide during the time interval Δ t (that is, t 2 − t 1). Since the reactant concentration decreases as the reaction proceeds, ΔH 2O 2 is a negative quantity; we place a negative sign in front of the expression because reaction rates are, by convention, positive quantities. Provides an example of data collected during the decomposition of H 2O 2. This behavior indicates the reaction continually slows with time. Using the concentrations at the beginning and end of a time period over which the reaction rate is changing results in the calculation of an average rate for the reaction over this time interval. At any specific time, the rate at which a reaction is proceeding is known as its instantaneous rate.
The instantaneous rate of a reaction at “time zero,” when the reaction commences, is its initial rate. Consider the analogy of a car slowing down as it approaches a stop sign. The vehicle’s initial rate—analogous to the beginning of a chemical reaction—would be the speedometer reading at the moment the driver begins pressing the brakes ( t 0). A few moments later, the instantaneous rate at a specific moment—call it t 1—would be somewhat slower, as indicated by the speedometer reading at that point in time. As time passes, the instantaneous rate will continue to fall until it reaches zero, when the car (or reaction) stops. Unlike instantaneous speed, the car’s average speed is not indicated by the speedometer; but it can be calculated as the ratio of the distance traveled to the time required to bring the vehicle to a complete stop (Δ t). Like the decelerating car, the average rate of a chemical reaction will fall somewhere between its initial and final rates.
The instantaneous rate of a reaction may be determined one of two ways. If experimental conditions permit the measurement of concentration changes over very short time intervals, then average rates computed as described earlier provide reasonably good approximations of instantaneous rates. Alternatively, a graphical procedure may be used that, in effect, yields the results that would be obtained if short time interval measurements were possible. If we plot the concentration of hydrogen peroxide against time, the instantaneous rate of decomposition of H 2O 2 at any time t is given by the slope of a straight line that is tangent to the curve at that time.
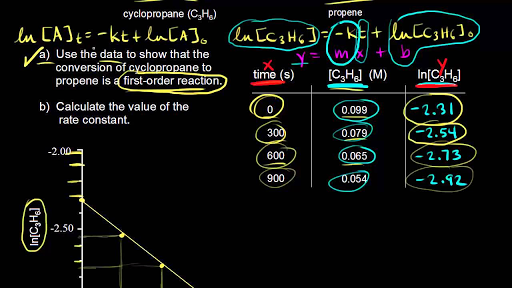
We can use calculus to evaluating the slopes of such tangent lines, but the procedure for doing so is beyond the scope of this chapter. Reaction Rates in Analysis: Test Strips for Urinalysis Physicians often use disposable test strips to measure the amounts of various substances in a patient’s urine. These test strips contain various chemical reagents, embedded in small pads at various locations along the strip, which undergo changes in color upon exposure to sufficient concentrations of specific substances. The usage instructions for test strips often stress that proper read time is critical for optimal results. This emphasis on read time suggests that kinetic aspects of the chemical reactions occurring on the test strip are important considerations. The test for urinary glucose relies on a two-step process represented by the chemical equations shown here.

The first equation depicts the oxidation of glucose in the urine to yield glucolactone and hydrogen peroxide. The hydrogen peroxide produced subsequently oxidizes colorless iodide ion to yield brown iodine, which may be visually detected. Some strips include an additional substance that reacts with iodine to produce a more distinct color change. The two test reactions shown above are inherently very slow, but their rates are increased by special enzymes embedded in the test strip pad. This is an example of catalysis, a topic discussed later in this chapter. A typical glucose test strip for use with urine requires approximately 30 seconds for completion of the color-forming reactions. Reading the result too soon might lead one to conclude that the glucose concentration of the urine sample is lower than it actually is (a false-negative result).
Tax Knowledge Assessment Study Guide
Waiting too long to assess the color change can lead to a false positive due to the slower (not catalyzed) oxidation of iodide ion by other substances found in urine. Test strips are commonly used to detect the presence of specific substances in a person’s urine. Many test strips have several pads containing various reagents to permit the detection of multiple substances on a single strip. (credit: Iqbal Osman) Relative Rates of Reaction The rate of a reaction may be expressed in terms of the change in the amount of any reactant or product, and may be simply derived from the stoichiometry of the reaction. Consider the reaction represented by the following equation. Chemistry End of Chapter Exercises.
What is the difference between average rate, initial rate, and instantaneous rate?. Ozone decomposes to oxygen according to the equation. Write the equation that relates the rate expressions for this reaction in terms of the disappearance of O 3 and the formation of oxygen. In the nuclear industry, chlorine trifluoride is used to prepare uranium hexafluoride, a volatile compound of uranium used in the separation of uranium isotopes. Chlorine trifluoride is prepared by the reaction. Write the equation that relates the rate expressions for this reaction in terms of the disappearance of Cl 2 and F 2 and the formation of ClF 3. A study of the rate of dimerization of C 4H 6 gave the data shown in the table: Time (s) 0 1600 3200 4800 6200 C 4H 6 ( M) 1.00 × 10 −2 5.04 × 10 −3 3.37 × 10 −3 2.53 × 10 −3 2.08 × 10 −3 Table 1.
Health Assessment Study Guide
(a) Determine the average rate of dimerization between 0 s and 1600 s, and between 1600 s and 3200 s. (b) Estimate the instantaneous rate of dimerization at 3200 s from a graph of time versus C 4H 6. What are the units of this rate? (c) Determine the average rate of formation of C 8H 12 at 1600 s and the instantaneous rate of formation at 3200 s from the rates found in parts (a) and (b). A study of the rate of the reaction represented as gave the following data: Time (s) 0.0 5.0 10.0 15.0 20.0 25.0 35.0 A ( M) 1.00 0.952 0.625 0.465 0.370 0.308 0.230 Table 2.
(a) Determine the average rate of disappearance of A between 0.0 s and 10.0 s, and between 10.0 s and 20.0 s. (b) Estimate the instantaneous rate of disappearance of A at 15.0 s from a graph of time versus A.
Chapter Assessment Reaction Rates Study Guided
What are the units of this rate? (c) Use the rates found in parts (a) and (b) to determine the average rate of formation of B between 0.00 s and 10.0 s, and the instantaneous rate of formation of B at 15.0 s. Consider the following reaction in aqueous solution: If the rate of disappearance of Br –( aq) at a particular moment during the reaction is 3.5 × 10 −4 M s −1, what is the rate of appearance of Br 2( aq) at that moment? Solutions Answers to Chemistry End of Chapter Exercises 1. The instantaneous rate is the rate of a reaction at any particular point in time, a period of time that is so short that the concentrations of reactants and products change by a negligible amount. The initial rate is the instantaneous rate of reaction as it starts (as product just begins to form). Average rate is the average of the instantaneous rates over a time period.
(a) average rate, 0 − 10 s = 0.0375 mol L −1 s −1; average rate, 12 − 18 s = 0.0225 mol L −1 s −1; (b) instantaneous rate, 15 s = 0.0500 mol L −1 s −1; (c) average rate for B formation = 0.0188 mol L −1 s −1; instantaneous rate for B formation = 0.0250 mol L −1 s −1.
Can you find your fundamental truth using Slader as a completely free Chemistry Matter and Change solutions manual? Now is the time to redefine your true self using Slader’s free Chemistry Matter and Change answers. Shed the societal and cultural narratives holding you back and let free step-by-step Chemistry Matter and Change textbook solutions reorient your old paradigms. NOW is the time to make today the first day of the rest of your life. Unlock your Chemistry Matter and Change PDF (Profound Dynamic Fulfillment) today.
YOU are the protagonist of your own life. Let Slader cultivate you that you are meant to be!
